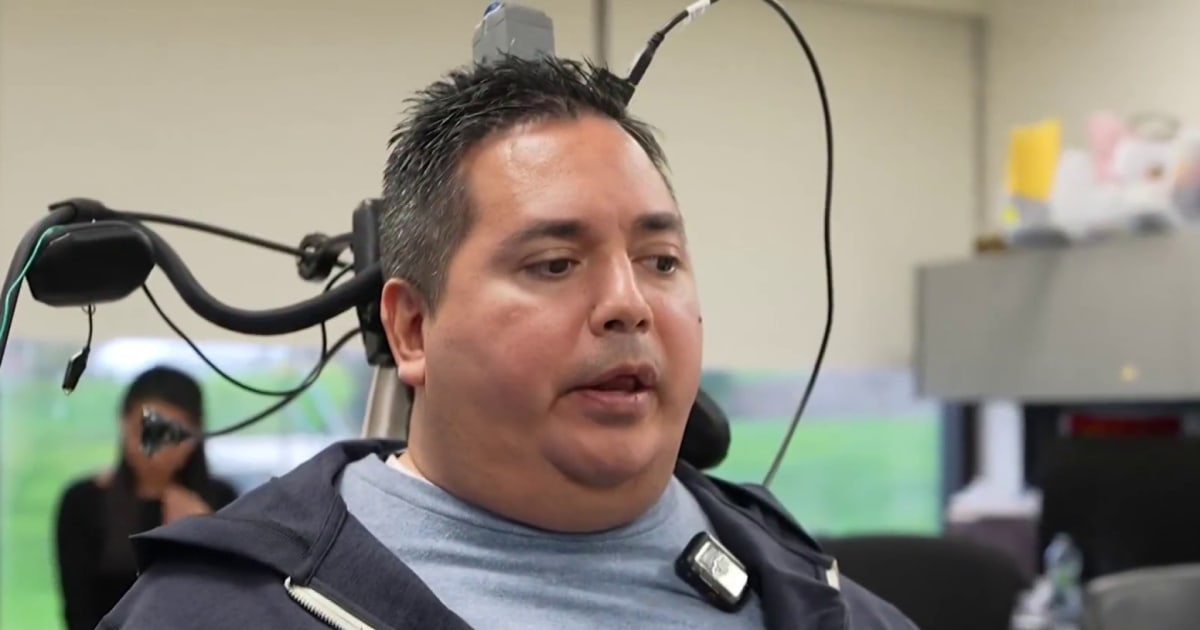
In a groundbreaking advancement for medical science, researchers at Northwell Health in New York have unveiled a new technology that enables paralyzed patients to regain movement in their limbs through neural implants. The innovation, detailed in a recent report from NBC News, represents a significant step forward in neurotechnology, offering hope to millions affected by spinal cord injuries and other forms of paralysis worldwide.
The technology involves surgically implanted devices that interface directly with the nervous system, bypassing damaged areas of the spinal cord to restore voluntary control over muscles. According to the NBC News video report, scientists at Northwell Health's Feinstein Institutes for Medical Research led the development, collaborating with engineers and neurologists to refine the implant's precision and safety. The project, which began several years ago, has now reached a stage where initial human trials are showing promising results.
One of the key breakthroughs came during trials conducted at Northwell's facilities in Manhasset, New York, where patients with complete paralysis below the injury site were able to perform basic movements, such as grasping objects or flexing fingers, for the first time in years. Dr. Ashesh Mehta, a neurosurgeon involved in the research, described the moment in the report: "It was extraordinary to see a patient who had been told they'd never move again suddenly control their hand with their thoughts." This quote underscores the emotional and scientific impact of the technology.
Northwell Health, the largest health system in New York, has been at the forefront of neuromodulation research for over a decade. The institution's labs have previously contributed to advancements in deep brain stimulation for Parkinson's disease and epilepsy. This new neural implant builds on that foundation, using advanced electrodes that detect and stimulate neural signals with unprecedented accuracy. The devices are powered wirelessly and designed for long-term implantation, minimizing the need for frequent surgeries.
Paralysis affects approximately 5.4 million people in the United States alone, according to estimates from the Christopher & Dana Reeve Foundation. Causes range from traumatic injuries, like car accidents or falls, to degenerative diseases such as multiple sclerosis. Traditional treatments have focused on rehabilitation and assistive devices, but they often fall short in restoring natural movement. The Northwell technology aims to address this gap by reconnecting the brain's motor commands directly to the muscles.
In the NBC News coverage, a patient participant, identified only as "John" to protect his privacy, shared his experience. "I felt my fingers twitch, and then I could actually pick up a cup," he said, his voice filled with awe during the interview. John's injury occurred in a 2018 motorcycle accident that left him quadriplegic. After undergoing the implant procedure in early 2023, he reported gradual improvements over several months of calibration and therapy sessions.
The procedure itself is minimally invasive compared to earlier experimental implants. Surgeons make a small incision in the abdomen to place the device, which then sends signals via thin wires to the targeted nerves. Post-surgery, patients undergo intensive training with physical therapists to recalibrate the system to their unique neural patterns. Northwell officials emphasized that while the technology is experimental, safety protocols have been rigorously tested in animal models and early human phases.
Experts outside Northwell have praised the development but cautioned about challenges ahead. Dr. Gregoire Courtine, a neuroscientist at the Swiss Federal Institute of Technology in Lausanne who has worked on similar projects, noted in a separate interview with NBC News affiliates: "This is a vital advancement, but scalability and affordability will determine its real-world impact." Courtine's team has developed complementary exoskeleton technologies, highlighting a potential synergy between implant-based and wearable solutions.
Funding for the Northwell project came from a mix of federal grants, including those from the National Institutes of Health, and private donations through the Northwell Health Foundation. The total investment exceeds $10 million, with additional support from tech partners like Medtronic, a leader in medical devices. This collaboration has accelerated the integration of artificial intelligence algorithms that help the implants learn and adapt to user intentions over time.
Broader context reveals a surge in neurotech innovations globally. Companies like Neuralink, founded by Elon Musk, are pursuing similar brain-computer interfaces, though their focus is more on broader applications like thought-controlled computing. In contrast, Northwell's approach is tailored specifically to motor restoration for paralysis. A 2022 study published in Nature reviewed over 20 such trials worldwide, finding that neural implants improved mobility in 70% of participants, though long-term data remains limited.
Critics, including some bioethicists, have raised concerns about accessibility. "Not everyone can afford or access cutting-edge surgery like this," said Dr. Mildred Cho, a bioethics professor at Stanford University, in comments echoed in media reports. She advocated for policies ensuring equitable distribution, especially since initial trials are limited to major medical centers. Northwell responded by stating plans to expand trials to diverse populations across the U.S. by 2025.
The implications extend beyond individual patients to societal shifts. Restoring mobility could reduce healthcare costs associated with lifelong care for paralysis, estimated at $1 million per person over a lifetime by the Reeve Foundation. It might also inspire innovations in treating other neurological conditions, such as stroke recovery or ALS. As one researcher put it in the NBC report: "This isn't just about walking again; it's about reclaiming independence."
Looking ahead, Northwell Health anticipates FDA approval for wider use within the next two years, pending successful completion of phase II trials involving 50 patients. Interim results, shared at a neurology conference in Boston last month, showed 80% of participants achieving at least partial limb control after six months. The team is also exploring applications for upper-body paralysis, which affects breathing and arm function.
In Appleton, Wisconsin, where local hospitals like ThedaCare are monitoring these developments closely, medical professionals expressed optimism. Dr. Sarah Linden, a neurologist at ThedaCare Regional Medical Center, said: "Advancements like this could transform care for our patients here in the Fox Valley. We're eager to see how it rolls out nationally." Appleton's proximity to research hubs in Madison and Milwaukee positions it well for potential adoption.
As the field evolves, ongoing debates about regulation and ethics will shape its trajectory. For now, the story from Northwell Health stands as a beacon of progress, reminding us of science's power to defy the once-impossible. Patients like John, and the millions waiting for breakthroughs, embody the human drive behind these innovations.